The Top 10 scientific surprises of Science News’ first 100 years
From the day Archimedes cut his bath short to shout “Eureka,” science has been a constant source of surprises.
Even after the abundant accumulation of knowledge in the intervening two millennia, science still retains the capacity to astonish, and the century since Science News began reporting has produced its share of shocking discoveries. Some such surprises happened suddenly (if not necessarily with eureka moments); in other cases, revolutionary shifts in understanding took a while to seep slowly into general scientific awareness.
In either case, Science News was sooner or later on the job during the last 100 years, identifying and reporting the never-ending series of surprises, too numerous to mention here, except for my Top 10.
10. Parity violation
In the 20th century, physicists established the importance of mathematical symmetries in the laws of nature. While all sorts of changes occur in the physical world, the equations describing them remain the same. So it seemed obvious that viewing the universe in a mirror — switching left and right — should have no effect on the accuracy of those equations. Hermann Weyl, a prominent mathematician who died in 1955, boldly stated that “there can be no doubt that all natural laws are invariant with respect to an interchange of right with left.”
But then in 1956 physicists Tsung-Dao Lee and Chen Ning Yang published a theoretical paper suggesting otherwise, and almost immediately two teams of experimenters showed that nature did indeed distinguish left from right (in technical terms, violating parity). Radioactive beta decay of cobalt atoms and the decay of unstable particles called muons both exhibited a left-right disparity in the directions traveled by the emitted beta particles — a major surprise. “It was socko!” recalled Leon Lederman, one of the experimenters, in an interview four decades later. “New atomic matter laws” proclaimed the headline in Science News Letter, the predecessor to Science News, with the subhead declaring the results “a revolution in theoretical physics.”
9. Inert gases make compounds
In the 1890s, chemists added a whole new family of elements to Dmitrii Mendeleev’s periodic table — the inert gases. Helium (detected on the sun decades earlier but not on Earth until 1895), neon, argon, xenon, krypton and radon had been previously missed because they did not — as far as anybody could tell — make compounds with other elements. Those reaction-resistant atoms became known as the inert or noble gases, as under ordinary conditions they all existed in the gaseous state. Textbooks all taught that the arrangement of electrons around the inert gas atoms precluded any opportunity for chemical combination.
Yet in 1962, a Science News Letter headline proclaimed “‘Impossible’ compound made with inert gas.” That article reported a xenon compound, xenon tetrafluoride, created at Argonne National Laboratory in Illinois, while acknowledging that earlier in 1962 chemist Neil Bartlett had already prepared another xenon compound, xenon-platinum hexafluoride. Chemists had to scramble to revise their textbooks, and scientists everywhere were reminded that you shouldn’t always believe what you’re told.
8. Plate tectonics
In the 1960s, many earth scientists were stunned to learn that the textbooks describing the planet’s history needed to be thrown away. Alfred Wegener, however, would not have been so surprised. Wegener, who died in 1930, was an astronomer-turned-meteorologist who dabbled in paleontology and geophysics. In 1915 he wrote a book proposing that the Earth’s continents had once been assembled in a single land mass, called Pangaea; they then, over millions of years, drifted apart to their positions on today’s world map. That map is not a permanent portrait of the Earth’s features, Wegener contended, but rather a snapshot snagged from a long-running movie. But few people believed Wegener, and geophysicists argued that such large-scale motion of such huge rigid masses was physically inexplicable. Wegener’s idea of continental drift did not die, though. Geologists knew all about it. But it remained heresy until the 1960s, when magnetic patterns detected on the seafloor suggested that oceans had expanded, pushing the continents away from one another.
“New evidence … supports the long-debated theory that continents were once connected and have drifted apart,” Science News Letter reported in 1963. Further work over the next few years showed that continental drift was a symptom of elaborate mechanisms inside the Earth that came to be known as plate tectonics. Plate tectonics explains not only the locations of the continents, but also how mountain ranges form and why earthquakes cluster in well-delineated zones of seismic activity. By 1969, Science News quoted experts declaring that it was time for “plate tectonics to be accepted as a basic theoretical model in geophysics.” And while many authorities for years remained reluctant to accept it, the decades following confirmed the surprising conclusion that Wegener had been essentially correct.
7. DNA makes genes
One of the last century’s most dramatic discoveries came in 1953, when James Watson and Francis Crick, aided by an X-ray image produced by Rosalind Franklin, figured out the double helix structure of the genetic molecule DNA. But perhaps the bigger surprise came a few years earlier, when Oswald Avery and colleagues at Rockefeller University in New York City showed that DNA was the substance that genes are made from. Although the reality of genes had been established in the early years of the 20th century, nobody had any good evidence about their physical structure. In the 1920s, “people were just as vague about what genes were … as they are now about consciousness,” Crick said in a 1998 interview. “The more professional people in the field … thought that it was a problem that was too early to tackle.”
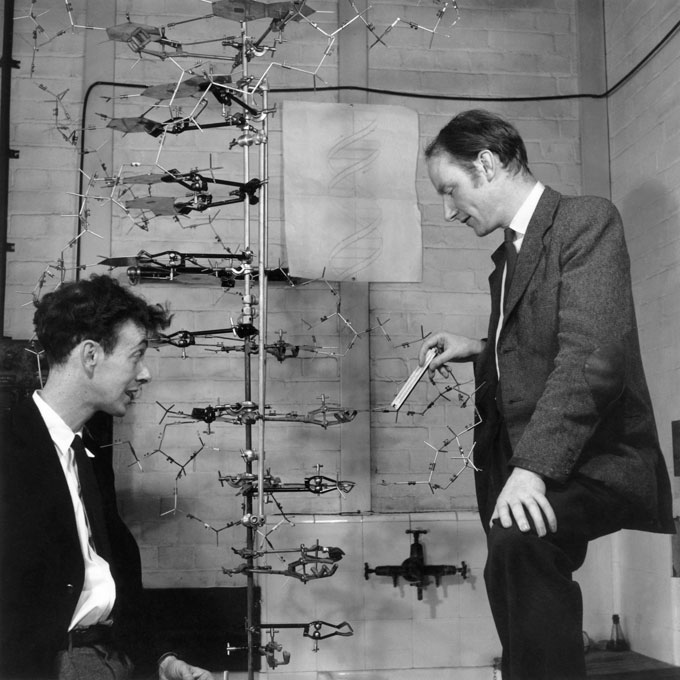
By the 1940s, the predominant view was that genes must be constructed from proteins of some sort. DNA was just an obscure organic acid. But in 1944, Avery and colleagues demonstrated that genes consisted of strands of DNA, not proteins. Science News Letter, however, apparently did not notice the Avery paper, citing instead two subsequent studies, in 1950 and 1952, confirming DNA as the genetic material. In 1953, though, Science News Letter recognized the DNA double helix structure as the top science story of the year. “Way life is handed on” headlined the story on Watson and Crick’s proposal for how DNA replication serves as the basis for heredity.
6. Dark energy
By the 1990s, the Big Bang theory of the expanding universe had been established beyond reasonable doubt, but questions remained. Chief among them was the fate of the universe. Most experts believed that the gravitational pull of mass throughout the universe was slowing its expansion down. But they debated whether there was enough mass to reverse the expansion altogether, shrinking the cosmos into a “big crunch.” Some thought the universe would expand forever, if at an ever-diminishing rate.
The plot in that story twisted rather shockingly in 1998, when two teams of astronomers reported measurements of light from distant supernovas. Those reports, subsequently bolstered by additional data, revealed that universal expansion was not slowing, but accelerating. Some repulsive force, nicknamed “dark energy” in the absence of firm knowledge of its true nature, apparently pervades the cosmos. Researchers were “stunned to find that the cosmos was expanding 10 to 15 percent more slowly in the past than can be accounted for” without a repulsive force today, Science News reported.
5. Dark matter
In the 1930s, physicist-astronomer Fritz Zwicky noticed that the velocities of galaxies moving within a group called the Coma cluster seemed to defy expectations based on the gravitational effects of the visible mass. Zwicky concluded that some unseen matter — he called it dunkle Materie, or “dark matter” — must be lurking in the cluster to reconcile the observations with the law of gravity. Later astronomer Horace Babcock and others noticed a similar discrepancy in the outer reaches of some galaxies: Stars revolved around a galaxy’s outer edges much faster than allowed by the galaxy’s apparent mass. In the 1970s and thereafter, astronomer Vera Rubin and collaborators confirmed the rapid velocity of the outer stars in many galaxies. As Science News reported in 1994, “Such behavior is a dead giveaway that the visible disk of these galaxies lies embedded in a much larger and more massive halo of unseen material.”

While the realization that most of the universe’s matter couldn’t be seen was surprising enough, an even greater surprise came when several lines of evidence affirmed that the dark matter could not be of the same type of matter known on Earth, composed primarily of protons and neutrons. Dark matter’s actual identity remains a mystery to this day; physicists have proposed some well-motivated possibilities, but the prospect remains that dark matter’s true nature will also come as a surprise.
4. Atomic bomb and nuclear fission
From the time of the discovery of radioactivity, physicists had speculated on the hidden energy packed into every piece of matter. And after Einstein published his famous equation E = mc2, it was clear that the amount of that energy would be enormous. But most experts doubted there would be any practical way to release such energy for useful purposes, or warfare weaponry. But in late 1938, chemists Otto Hahn and Fritz Strassmann found that experiments bombarding uranium with neutrons produced evidence of the much lighter element barium. Lise Meitner (who had collaborated with Hahn before fleeing Nazi Germany) and her nephew Otto Frisch figured out what had happened — the uranium nucleus had been split into pieces. Frisch immediately told Niels Bohr, about to board a ship for America. And soon after Bohr arrived, the word was out. “Atomic energy released” headlined a Science News Letter cover story in early 1939, while reassuring its readers that “physicists are anxious that there be no public alarm over the possibility of the world being blown to bits by their experiments.”
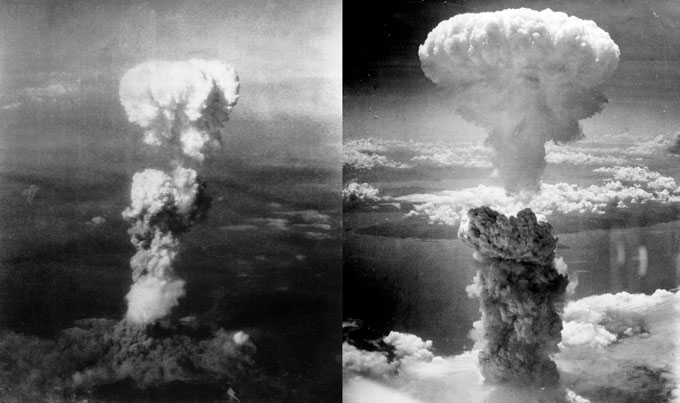
Soon, though, nuclear fission was transformed into a massive war project for building an unimaginably powerful explosive, shocking the world with its potential for destruction, while offering only partially fulfilled hope for a reliable source of useful energy. “Doomsday thunderbolts,” as Science News Letter labeled the atomic bombs dropped on Japan, “herald a revolution in war such as has not been seen since the first use of gunpowder, and later on another revolution in industry probably greater than the one ushered in by the invention of the steam engine.”
3. Expanding universe
Philosophers and physicists alike had long pondered deep questions about the nature of the universe — whether it was finite or infinite, for instance, or whether it had a beginning or had existed eternally. But just about everybody believed that on the whole it never changed, but rather just existed, its objects rotating and revolving in cycles that forever repeated. Only a rare few (the poet Edgar Allan Poe, for example) had imagined an evolving, changing universe. But in the 1920s, mathematician Alexander Friedmann suggested that the universe might be growing or shrinking, based on his solutions to the equations of Einstein’s general theory of relativity. Einstein himself had noticed that possibility earlier, but altered his equations so that they would predict an unchanging universe, as he knew of no evidence that it was otherwise.
But such evidence was already being collected, in measurements of the colors of light emitted by distant nebulae (later called galaxies). Analysis of that data led Edwin Hubble to show, in 1929, that the farther away a galaxy was, the faster it was flying away — implying (although Hubble didn’t immediately agree) that the universe is in fact expanding. “The distant nebulae are rushing away from us at tremendous speeds and thus the real universe is constantly expanding,” Science News Letter reported in 1931.
2. Antimatter
In 1930, Science News-Letter reported on an incredibly bold proposal by a young British physicist named Paul Dirac. He argued that matter — the solidity from which physical objects are constructed — was in fact nothing more than a bunch of “holes” in the vacuum of space. Space, he suggested, is not empty, but rather completely full of electrons endowed with “negative energy.” Those negative energy electrons could not be detected. But in spots where a negative-energy electron had been given enough energy to lift it from the negative-energy sea, a hole would form, like an empty bubble in an ocean. The absence of the electron would make the hole appear to have a positive electric charge.
Dirac presumed such positively charged bubbles in the negative-energy ocean would correspond to protons, the fundamental particle making up the bulk of the mass of all atoms — in other words, all matter. But that turned out not to be the surprise, because Dirac was wrong. He soon realized that the positively charged holes could not be protons, but rather must be much lighter, with the same mass as an ordinary negatively charged electron. Dirac thus predicted the existence of antimatter, an entirely novel idea. An ordinary electron meeting its antimatter particle would disappear by filling the hole, releasing a burst of energy in the process.
Dirac’s “anti-electron” was very shortly thereafter detected in cosmic rays by physicist Carl Anderson, who reported “the probable existence of a … positively charged particle of the mass of the familiar negative electron.”
1. Uncertainty principle
In 1927, Werner Heisenberg announced his uncertainty principle, the core idea underlying the newborn math for describing nature known as quantum mechanics. Heisenberg’s principle expressed the shocking realization that the unbroken chain of cause and effect deduced from Newtonian physics was an illusion, an approximation that nature did not observe on the subatomic scale. It took a couple of years, but Science News-Letter proclaimed “‘Uncertainty principle’ enters science” in a 1929 headline. “Crudely stated, the new theory holds that chance rules the physical world,” the article announced. “These weird sounding consequences arise from the contention that a particle may have an exact place or an exact speed, but it can not have both.” The report called it a “disturbing idea” that would “revolutionize the ideas of the universe … to an even greater extent than Einstein’s relativity” and was sure to make a stir “once philosophers and laymen begin their attempts at its interpretation.” Such attempts continue today — and any future interpretation succeeding in forging a consensus will surely come as an especially unexpected surprise.
For all the latest Technology News Click Here
For the latest news and updates, follow us on Google News.
